Choosing and using rechargeable batteries
Looking to get rid of your alkaline throwaway battery habit? There’s a new kind of Nickel Metal Hydride battery in the shops, with so low a rate of self-discharge that they come factory charged and ready to use. Unfortunately some kinds of device can’t use them. Learn more…
There are some compelling reasons for using rechargeable batteries in your torches, flashguns, radios, digital cameras and so on, where you would currently use AA or AAA alkaline cells. Not only because in the long run you could save a lot of money, but also because you can help reduce the burden on the environment that is caused by one-time-use batteries.
The UK has a particularly dismal record when it comes to recycling batteries: government agencies reckon it is only between three and five percent. Untold millions and millions of old batteries in the UK end up in landfill, where they leak harmful chemicals into the soil, potentially into the water supply.
In contrast, a rechargeable battery will give good service for hundreds of charges if looked after well; and when it comes to the end of its life you should dispose of it properly — something that is now a lot easier to do thanks to recent legislation.
Nickel Cadmium cells: old & tricky, but have their uses

Rechargeable batteries are best kept ready for use in some form of protective container. I bought these boxes from a Maplin store: they can be used to contain either four AA cells or four AAA cells.
In this essay I am focussing on the kinds of rechargeable battery that typically replace an alkaline AA or AAA cell. So I am not talking about car batteries, which use lead-acid chemistry, nor the button cells that power hearing aids and watches, nor lithium-ion batteries — which are often custom designed to work with a particular kind of digital camera or camcorder.
The first kind of rechargeable alternative to the everyday alkaline battery to come along was the Nickel Cadmium cell (often abbreviated to NiCd or NiCad). In the 1980s and 1990s small NiCad batteries were the main kind of rechargeable battery used in consumer electronics. NiCad chemistry has the advantage over both alkaline and NiMH of being able to deliver a lot of power in a short time, so NiCad cells are the best choice for powering photographic flashguns. For the same reason, special high-power NiCad packs are used in cordless drills.
NiCads: the charge sheet…
These are the characteristics of NiCad batteries — some of them are problematic…
- If you are in the habit of using half of the power stored in a NiCad cell, then recharging it, the cell tends to ‘forget’ that it has all that capacity available to it, and so runs out of useful power more quickly the next time. This was a big problem when most mobile phones used NiCad battery packs. Rather than just have one set of batteries, it is best to have two or more, so that you can with confidence run the batteries right down, knowing you have a backup set. And NiCad cells don’t mind deep discharge: it is good for them!
- NiCad cells deliver a lower voltage than alkaline cells; between 1.25 and 1.35 volts, rather than the 1.5 volts that comes out of a fresh alkaline cell. This normally isn’t a problem when used with consumer equipment, because the voltage of an alkaline cell in actual use tapers off, until it is close to 0.9 volts when it is near exhaustion. As consumer gear is designed for use with alkaline cells, this lower NiCad voltage is therefore usually not a problem. (Unless a device is particularly sensitive to low voltage, see below…)
- NiCads pretty much deliver a constant voltage without the power taper of alkalines, then they fade very rapidly! That’s when you are glad you have a spare set. And when you spit blood because that DIY job is sabotaged by a suddenly exhausted electric drill!
- Once fully charged, a NiCad cell will start to lose its charge (‘self-discharge’) at a rate of 20% a month. This is a tricky problem, because if you use NiCads only occasionally, you could be tempted to top up the charge before use, which puts you at risk of encountering the ‘memory effect’ described above. It is recommended to store NiCad cells empty (run them right down in something), and charge them shortly before you need them. (Which takes more planning than most of us are good at!)
- Nickel Cadmium chemistry is pretty toxic, and an environmental menace if the batteries are not properly disposed of.
Nickel Metal Hydride – old style and new

Various brands of the new-style ‘hybrid’ NiMH cells, which have a low self-discharge rate and are best kept charged and ready for use.
Nickel Metal Hydride batteries started to appear at the beginning of the 1990s. They used the same nickel oxyhydroxide positive electrode as NiCads, but the toxic cadmium is replaced by a metal alloy, generally of titanium and nickel. The abbreviation is NiMH (and has nothing to do with America’s National Institutes of Mental Health, just in case you Googled for that and got here by mistake).
The capacity of NiMH cells is excellent, two or three times the capacity of NiCad cells of the same size. Incidentally, the way to compare the capacity of batteries is to look for the mAh figure that is generally printed on the battery – it stands for ‘milliAmp hours’, and the higher the number, the more juice it stores when charged.
When I started buying and using NiMH cells around the turn of the century, the thing I most appreciated about them compared with NiCads is that they did not exhibit that irritating ‘memory effect’ — it is quite OK to top them up when they are only half-discharged.
On the other hand, until 2005 all the available NiMH batteries had quite a woefully high rate of self-discharge. In fact they can lose 10% of their charge on the first day, and a further 1% a day after that, and before long your ‘2700 mAh’ battery has only half its charge left.
LSD cells: buy these precharged
In 2005, Sanyo introduced a new kind of NiMH cell, the LSD NiMH or Low Self Discharge NiMH cell, marketed as ‘eneloop’. Other manufacturers followed on, and I have bought batteries of this type as Uniross ‘Hybrio’, Camelion ‘AlwaysReady’, Hahnel ‘Synergy’ and Tronic ‘Precharged’.
With a differently formulated positive electrode and an improved separator, these batteries lose only between 10% and 20% of their charge over a period of a year! Therefore they are cleverly marketed as having been factory pre-charged, and supplied to you from the shop ready to use.
Other benefits that come from the new formula are that with less internal resistance, this kind of NiMH cell doesn’t get so hot while charging, and is better able to deliver peak power demand than the old sort. It is true that they tend to have lower overall capacity than the older style cells (the AA size is typically 2100 mAh, the AAA 900 mAh), but remember that unless you used those high-capacity cells straight out of the charger, part of that capacity had already gone.
In general, LSD NiMH cells are not cheap! A set of four AA cells typically costs in the region of nine or ten quid. However, it pays to keep your eyes peeled. One weekend I was cruising the discount hardware section of a Lidl supermarket, and found sets of Tronic LSD NiMH at £2.99 a pack of four. Well, I bought three packs!
When devices don’t let you be green…
Just when you thought you could Go for Green and say goodbye to the alkaline disposable battery, you find that some of your technology doesn’t want to play along. I once borrowed a cheap Vivitar digital camera for an evening, and popped in two freshly-charged LSD NiMH cells. It took three pictures, then shut down with a flashing ‘battery drained’ indicator. Why?
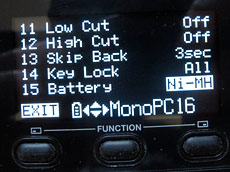
When setting up a Preset on the Marantz PMD661 recorder, you can tell it what kind of batteries you are using.
On reflection, I realised what was going on. The designer of that device set it up to monitor the voltage of the batteries, on the assumption that alkaline cells would be in use, delivering 1.5 volts when fresh and tapering from there. As far as the Vivitar was concerned, the 1.2 volts it measured from my cells indicated a set of alkalines dipping below its voltage threshold, and it shut down in self-preservation.
Similarly, I have a Sony XDR-S55DAB portable Digital Audio Band radio; DAB radios are typically rather power hungry and I run it off its transformer, but it can take four AA cells. But it too has a low tolerance for low voltage; even with a freshly charged set of NiMH cells inserted, it won’t even wake up. Much as I like Sony I have to say this: if you are looking for a DAB radio manufacturer with environmental awareness, chose Roberts instead.
The most ‘educated’ device I own when it comes to playing along with NiMH rechargeables is my Marantz PMD661 audio recorder. I was most impressed when I found that in the long list of preferences in the Presets menu, you can tell it whether you are using Alkaline or NiMH batteries, and it will monitor the battery discharge profile appropriately. Now that’s what I call civilised!
Care, feeding and disposal of NiMH batteries

My home-made ‘battery sock’, a wide piece of elastic, machine-stitched. Why doesn’t someone make these commercially?
- How to charge. You should charge any kind of NiMH battery in a charger which is specifically designed to know about these kinds of cells. NiMH battery chargers mostly use the ‘delta V’ method, measuring the rate of change of the voltage of the batteries as they charge up. Charging is done quite rapidly at a constant current, and just when the cell is full and begins to overcharge, it displays a ‘reverse polarity’ effect which results in a slight drop of voltage. At this point, a properly-designed charger will stop the charging cycle. There is usually some kind of LED signal that indicates when the charge cycle is complete.
- Service life. The old-style NiMH cells are good for about 500 recharges; the new-style LSD type can manage about 1000 charges, which helps to compensate for their higher cost.
- Over-discharge. If a NiMH cell is completely discharged, it can go into polarity reversal which if continued for any appreciable length of time will permanently damage the cell. A Wikipedia article suggests that this could occur if you have a number of cells in series (e.g. four in a row) and one exhausts before the others. If that happens, the other cells will drive the exhausted cell in reverse and damage it. Most digital devices would have shut down well before that point, but there is some risk if you have a set of cells in a ‘dumb’ device such as a torch. I recommend keeping cells in ‘teams’ which grow old together; and change and feed them before they get too tired.
- Disposal. When you judge that it is time to get rid of rechargeable cells, don’t throw them in the bin! By law, any retail outlet that sells more than 36 kg of batteries now has to provide a point where used ‘portable’ batteries (i.e. not industrial or car batteries) of all types are collected for recycling. Try a large supermarket! This is as true of NiCad, NiMH, mercury cell and Lithium-ion batteries as it is for alkalines. The batteries will be dismantled, and nickel in particular can be reclaimed from them.

